Abstract
Background: Ibrutinib is an oral once-daily Bruton's tyrosine kinase inhibitor (BTKi) and is the only targeted treatment that has demonstrated significant progression-free survival (PFS) and overall survival (OS) benefit in multiple phase 3 trials for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). With ibrutinib established as a preferred first-line (1L) treatment option, it is important to better understand the treatment sequence after 1L ibrutinib and the characteristics of patients who are treated with a second-line (2L) therapy. Using a real-world US administrative claims database, this study aimed to compare time to next treatment (TTNT), healthcare resource utilization (HRU), and healthcare costs between different 2L treatment regimens (cohorts) used after ibrutinib.
Methods: Adults with CLL/SLL treated with 1L ibrutinib were identified in a US claims database (Optum's de-identified Clinformatics ® Data Mart Database; 02/12/2013-03/31/2020). Those receiving a 2L therapy were considered eligible for the study. Retreatment with ibrutinib, in-class switch to another BTKi, or treatment with anti-CD20 immunotherapy-only were not considered eligible 2L treatment options for this evaluation. The most commonly used treatment regimens were categorized into the following clinically-relevant treatment cohorts: 1) chemoimmunotherapy (CIT) or chemotherapy (CT)-only, and 2) venetoclax (VEN)- or idelalisib (IDELA)-based regimens (monotherapy or combination regimens). Comparisons of TTNT, HRU (inpatient admissions, outpatient visits, emergency room visits, other ancillary services), and per patient per month healthcare costs (inpatient admissions, outpatient visits, emergency room visits, other ancillary services, pharmacy) were evaluated based on the sum of the payer and patient paid amount during 2L treatment. Comparisons were adjusted using multivariate regression including confounders observed in the baseline period and during 1L ibrutinib therapy. For costs and HRU, 95% confidence intervals and p-values were obtained from 499 bootstrap resamples.
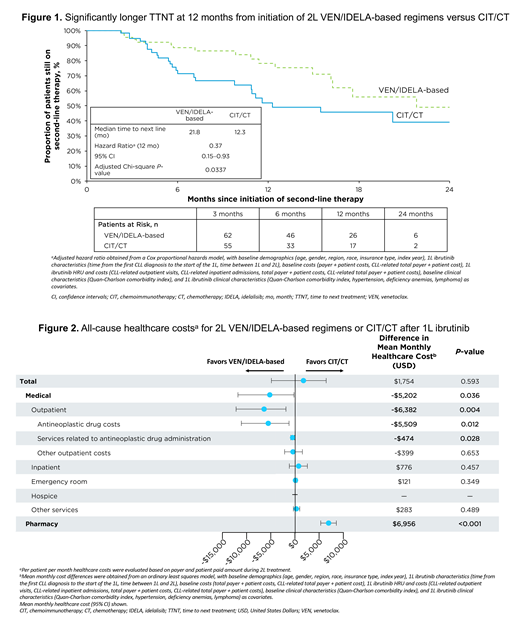
Results: A total of 132 CLL/SLL patients who received ibrutinib as a 1L treatment and had a subsequent eligible 2L therapy were included; mean age was 74 years old, 64% were male. Eligible patients received 2L CIT/CT-only (63/132, 48%), or VEN/IDELA-based regimens (69/132, 52%). TTNT was significantly longer for 2L VEN/IDELA-based regimens than for CIT/CT (hazard ratio at 12 months: 0.37; P=0.0337) (Figure 1). The number of outpatient visits for antineoplastic drug administration was significantly lower for patients receiving 2L VEN/IDELA-based regimens compared to CIT/CT (rate ratio=0.44; P=0.004). Total mean monthly healthcare cost (includes CLL/SLL and non-CLL/SLL-related medical/pharmacy costs) was $1,754 higher, but not significantly different (P=0.593), for patients receiving VEN/IDELA-based regimens compared with CIT/CT (Figure 2). Total mean monthly medical cost (non-pharmacy) was significantly lower ($-5,202; P=0.036) for patients receiving VEN/IDELA-based regimens compared to CIT/CT (Figure 2).
Conclusions: This study demonstrates that VEN/IDELA-based regimens may be a more viable treatment option post-1L ibrutinib use for CLL/SLL patients compared to CIT/CT. When compared to 2L CIT/CT, use of VEN/IDELA-based regimens post-1L ibrutinib use was associated with significantly longer TTNT and lower medical costs, signifying reduced disease burden to the patients and lessened burden to the healthcare system. These findings also suggest that treatment convenience associated with 2L oral targeted regimens may play an important role in improving treatment outcomes. These results support the need for evaluating treatment sequencing strategies in the real-world setting to further improve clinical outcomes and reduce the burden of total cost of care for patients with CLL/SLL.
Challagulla: Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Emond: Janssen: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; GlaxoSmithKline: Consultancy. Volodarsky: Pharmacyclics LLC, an AbbVie Company: Current Employment; AbbVie: Current equity holder in publicly-traded company. Young: AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Manceur: AbbVie: Consultancy; Actelion, part of the Janssen Pharmaceutical Companies of Johnson & Johnson: Consultancy; Bristol-Myers Squibb: Consultancy; Daiichi-Sankyo: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy. Karve: AbbVie: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal